Ice crystals: Some times I really can't help myself, so let's hear it from me.
The influence on food freezing: ice crystal behavior during fast/slow freezing and thawing of food
For the growth of ice crystals, there is a very important influencing factor - the rate of cooling. When slow cooling, because of the long time to cool down to below the freezing point of water in the food, the initial water molecules form nuclei that are easily dispersed by the thermal movement of other water molecules, resulting in a reduction in the number of stable nuclei formed. At the same time the long cooling process gives water molecules sufficient time to focus on the combination of a small number of stable nuclei, and so grew into large ice crystals; and when rapidly cooled, because the temperature quickly dropped to a supercooling temperature, immediately formed a large number of stable nuclei. At the same time, the water molecules do not have sufficient time to collect, so they can only be scattered to each stable nucleus, forming numerous small ice crystals.
Take to the food freezing level, there is a more professional term - freezing speed, it refers to the food surface and the shortest distance between the central temperature point, and the food surface to 0 ℃ after the central temperature of food down to 10 ℃ lower than the food freezing point of the ratio of the time required. The speed of freezing can generally be divided by the time the central temperature of the food falls or the distance the freezing layer extends. The central temperature of food from -1 ℃ down to -5 ℃ required time (i.e., through the time), within 30 min, is fast freezing, more than 30 min is slow freezing. Fast freezing often gives ice crystals in the form of needle-like rods with particle sizes ranging from 0.5 to 100 μm, while slow freezing often gives ice crystals in the form of cylindrical or block-like particles with particle sizes ranging from 100 to 1000 μm.
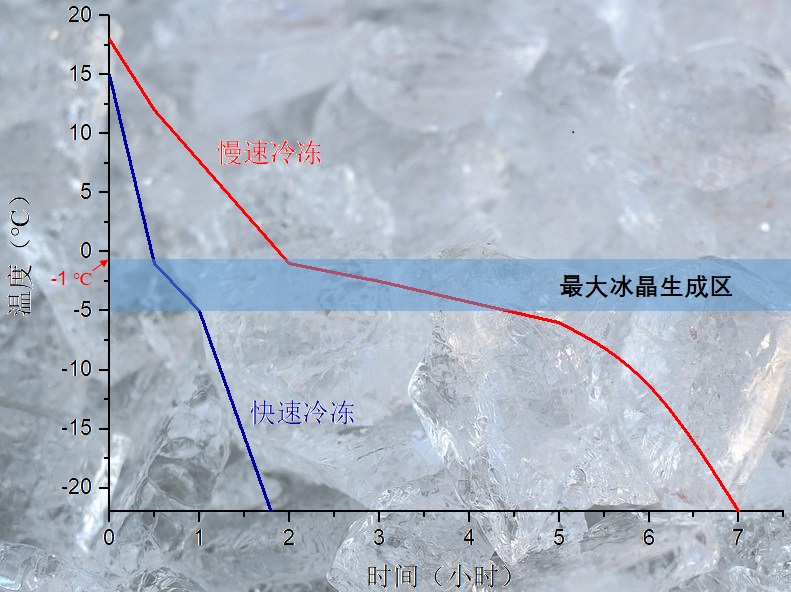
Above text: slow freeze, fast freeze, maximum ice crystal generation zone
All plants and animals are made up of cell membranes or cell walls surrounding the cells. Water is the main component of each organism. Water in plant and animal foods exists in two states inside the cell and in the intercellular space: one will be combined with proteins, sugars and other substances to participate in cell composition, called bound water, which has a very low freezing point but is not high; one exists in large quantities inside and outside the cell, called free water, which can be used as a medium and flows freely.
When slow freezing occurs, ice crystals initially form in the free water present in the intercellular space, followed by the bound water within the cell. As the ice crystals form, the concentration of the solution encompassing them increases, creating an osmotic pressure difference inside and outside the cell. At this stage, irreversible damage occurs, leading to cell membrane rupture caused by an excess amount of water precipitation outside the cell. The strengthened role of salt precipitation further exacerbates the irreversible denaturation of proteins. Therefore, it is crucial to do structure confirmation and react accordingly during slow freezing processes.
This condition of food thawing, ice crystals melt to form the free state of juice is difficult to be reabsorbed, the formation of "blood water", which has a large number of proteins, sugars and other nutrients. At the same time, because of food cell damage, it also lost the original form, nutrition and flavor.
Table 1 Relationship between freezing rate and ice crystal shape
Freezing speed through 0~-5℃ time | Ice Crystal Location | Ice crystal shape | Ice crystal size (diameter x length) | Number of ice crystals | Ice advancement speed(I) Water movement velocity(W) |
| seconds | Intracellular | Needle-shaped | 1~5×5~10μ | Numerous | I≫W |
1.5 minutes | Extracellular | Rod-shaped | 0~20×20~50μ | Majority | I>W |
| 40 minutes | Intracellular | Columnar | over 50~100×100μ | Few | I<W |
| 90 minutes | Extracellular | Block-shaped | over 50~200×200μ | Few | I<<W |
(Table from: Mao-Ping Zhang. Study on the characteristics of ice crystal formation in frozen foods[J]. Refrigeration, 1993(01):50-56.)
During the process of rapid freezing, ice crystal formation surpasses the velocity of water diffusion. This rapid crystallization development causes bound water, free water, and water present in the cell interstices to freeze almost simultaneously, resulting in numerous small ice crystals. This process hinders any increase in volume, subsequently preventing cell deformation or membrane rupture. Therefore, the cell protoplasm remains unharmed and intact.
When the food is thawed, the free juice formed by the melting ice crystals may be quickly absorbed to restore the cells to their original state, thus maximizing the color, flavor and nutrition of the food.
Ice crystals: So, I really do not mean to destroy human food, you do not just blame me, but to think of ways to help me control my size, to solve the problem at the root!
Subscribe to be the first to get the updates!