Oral solid drugs are widely used in the pharmaceutical industry due to good stability, cost-effectiveness, and convenient portability and administration. Oral drug absorption relies on dissolution as a prerequisite. Yet, literature indicates that around 40% of currently marketed drugs are characterized by poor solubility, with nearly 90% of candidates in research and development pipeline falling into this category. Consequently, enhancing drug solubility and improving bioavailability are pressing challenges in realm of new drug development. This article will delve into various widely employed method for solubility enhancement, including salt formation, polymorphism, particle size reduction of active pharmaceutical ingredients (APIs), the preparation of amorphous solid dispersions (ASD), and the addition of cyclodextrins or surfactants.
Before exploring the intricacies of each technique, it is crucial to grasp a significant concept —the Developability Classification System (DCS). This system serves as a guide for researchers, helping them discern whether a drug molecule requires solubilization and adding in the selection of appropriate solubilization method.
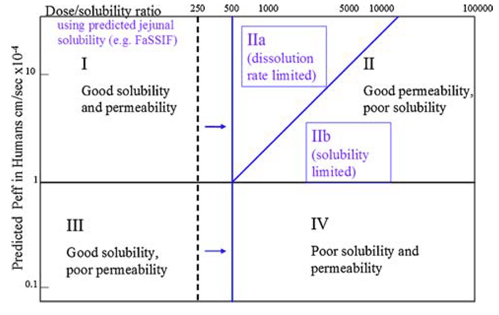
Similar to the Biopharmaceutics Classification System (BCS), the DCS categorizes drugs into four classes determined by their solubility and permeability. DCS refines the assessment criteria for solubility and permeability, and within Class II, it introduces further distinctions. Class II is subdivided into DCS IIa (absorption limited by dissolution) and DCS IIb (absorption limited by solubility), accounting for different factors that restrict in vivo absorption.
For drugs classified as DCS I and III, solubilization is not necessary, and conventional formulation technology can be adopted. In the case of DCS IIa compounds, addressing absorption challenges arising from dissolution an often be tackled through strategies like particle size reduction. However, for compounds classified as DCS IIb and DCS IV, alternative methods such as salt formation, preparation of metastable polymorphs, or ASD are recommended to enhance drug solubility and bioavailability.
DSC
Salt formation represents a straightforward and cost-effective method for solubilization. Organic weak acid or organic weak basic drugs can be converted into soluble salts, thereby increasing their solubility and improving oral bioavailability.
Literature indicates that around 50% of drugs in the U.S. market exist in salt forms. Examples include the anticancer drug Imatinib (Gleevec®), the antihypertensive drug Metoprolol (Lopressor®), and the anti-inflammatory and analgesic drug Diclofenac (Voltaren®). Specifically, Diclofenac, a nonsteroidal anti-inflammatory drug, has multiple salt forms such as sodium salt and potassium salt. While the solubility of free diclofenac in water is very low (approximately 0.02 mg/mL), the solubilities of the sodium salt and potassium salt are significantly higher (9.7 mg/mL and 4.6 mg/mL, respectively), leading to a substantial improvement in solubility.
Polymorphism is widely present in small molecule drugs, where the same compound, subjected to different crystallization conditions (solvent, temperature, cooling rate, etc.) and operational procedures (evaporation, stirring, anti-solvent addition, etc.), can adopt diverse molecular arrangements and lattice structures, resulting in polymorphs. Various polymorphs exhibit differences in lattice energies, melting points, dissolution rates, and solubilities. Typically, stable polymorphs exhibit lower Gibbs free energy, higher melting points, slower dissolution rates, and lower solubilities. Conversely, metastable polymorphs tend to have lower melting points, higher solubility, and faster dissolution rates.
Crystalline forms comprising multiple components encompass solvates and co-crystals. In the crystallization process, solvent molecules may permeate the crystal lattice, altering the crystal structure and giving rise to solvates. When water serves as the solvent, hydrates are generated. Typically, the hierarchy of solubility and dissolution rate in water is observed as follows: hydrate < anhydrate < solvate with an organic solvent.
As outlined in the FDA's "Regulatory Classification of Pharmaceutical Co-Crystals (2018)," co-crystals are crystalline substances where two or more distinct molecules, typically the active pharmaceutical ingredient (API) and co-crystal formers (coformers), are combined in predetermined stoichiometric ratios through non-ionic and non-covalent bonds within a unified crystal lattice. Distinct from salts, co-crystals are categorized as polymorphs, not novel APIs. They are employed to enhance drug solubility, bioavailability, and stability.
Micronization is a fluid dynamics-driven process that diminishes particle sizes to the micrometer range. In the context of APIs, micronization increases specific surface area, porosity, and surface energy of the drug particles. This, in turn, enhances the solubility and bioavailability of poorly soluble drugs. Micronization has evolved into a well-established API technology, finding successful applications in both clinical and commercial products. It is particularly well-suited for DCS IIa compounds.
An alternative approach particle size reduction involves the use of nanocrystals. Nanocrystal drugs are characterized by particle sizes in the nanometer range, typically below 1 μm (practically ranging from 200 to 500 nm). Formulations utilizing nanocrystals exhibit very small particle sizes, offering distinctive properties and benefits. These include heightened drug solubility, expanded surface area, improved bioavailability, and accelerated drug release, rendering them well-suited for DCS IIa compounds. However, this technology presents challenges, including intricate manufacturing processes and elevated production costs.
Amorphous Solid Dispersion (ASD) stands as well-established and extensively utilized formulation technology, recognized as one of the most effective strategies for bioavailability enhancement. Additionally, it proves adept at mitigating the impact of food on drug effects.
The rationale behind ASD is: by uniformly dispersing an insoluble drug in an amorphous form within a carrier, the dispersion, surface area and dissolution rate of the drug can be increased. This, in turn, further improves the solubility and bioavailability. Spray drying and hot melt extrusion are two commonly used methods for preparing ASD. To date, over thirty products have been successfully commercialized with ASD.
Cyclodextrins are cyclic oligosaccharides with a hydrophilic surface and a hydrophobic cavity possessing high water solubility. They have the capability to form inclusion complexes with hydrophobic solutes, effectively boosting the apparent solubility of these solutes. Furthermore, the complexation with cyclodextrin can alter the lipid barrier at absorption sites, facilitating drug absorption and augmenting bioavailability. This is attributed to the interaction of cyclodextrins with membrane components such as cholesterol, phospholipids, and proteins, thereby modifying the permeability of the lipid barrier.
Surfactants are amphiphilic molecules with both nonpolar hydrophobic groups and polar hydrophilic groups. The hydrophobic portion typically comprises hydrocarbon chains with a minimum of 8 carbon atoms, heterocycles or aromatic groups. The polar groups may exist as dissociated ions or undissociated hydrophilic groups, such as carboxylic acids and their salts.
Due to their distinctive structure, surfactants exhibit robust surface activity, allowing them to orient at the interface of two phases and reduce the surface tension of the liquids. Surfactants find frequent application in solubilization, with microemulsion being an illustrative example. Microemulsion is a transparent or translucent, low viscosity, isotropic and thermodynamically stable system formed by the appropriate proportion of water phase, oil phase, surfactant and cosurfactant. In the realm of oral drug delivery, microemulsions, utilizing surfactants as carriers, prove effective in overcoming the first pass effect and breaking the absorption barrier for macromolecule drugs. They can be absorbed through the lymph directly after oral administration.
Enhancing the solubility of poorly soluble drugs can be achieved through various viable methods. In practical terms, determining the appropriate formulation strategy requires consideration of factors such as drug properties, development stages, target doses, development costs.
1. Sandeep Kalepu et al. Insoluble drugdelivery strategies: review of recent advances and business prospects[J]. Acta Pharmaceutica Sinica B, 2015,5(5):442–453.
2. Evan A Thackaberry. Non-clinical toxicological considerations for pharmaceutical salt selection[J]. Expert Opin Drug Metab Toxicol, 2012,8(11):1419-1433.
3. James M. Butler et al. The Developability Classification System: Application of Biopharmaceutics Concepts to Formulation Development[J]. Journal of Pharmaceutical Sciences, 2010, 99(12): 4940-4954.
4. Book: Nanostructures for Oral Medicine. 2017.
5. Ron Liu. 《难溶性药物制剂技术》. 刘荣译. 北京:化学化工出版社,2021.
6. 田阳等.纳米晶体药物制备技术的研究进展[J]. 药学学报, 2021, 56(7): 1902-1910.
7. Pharmaceutical Co-Crystals: FDA Finalizes Guidance – RAPS.
8. 崔德福. 《药剂学》. 人民卫生出版社,第7版.
Subscribe to be the first to get the updates!